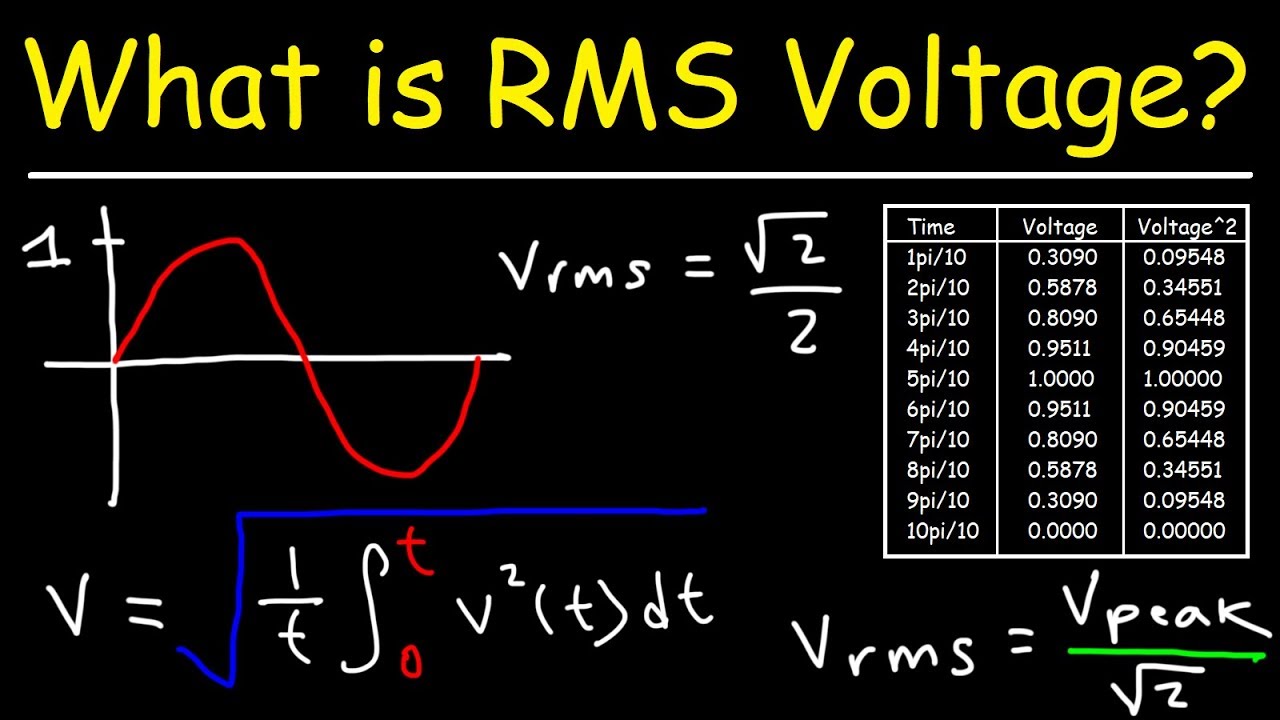
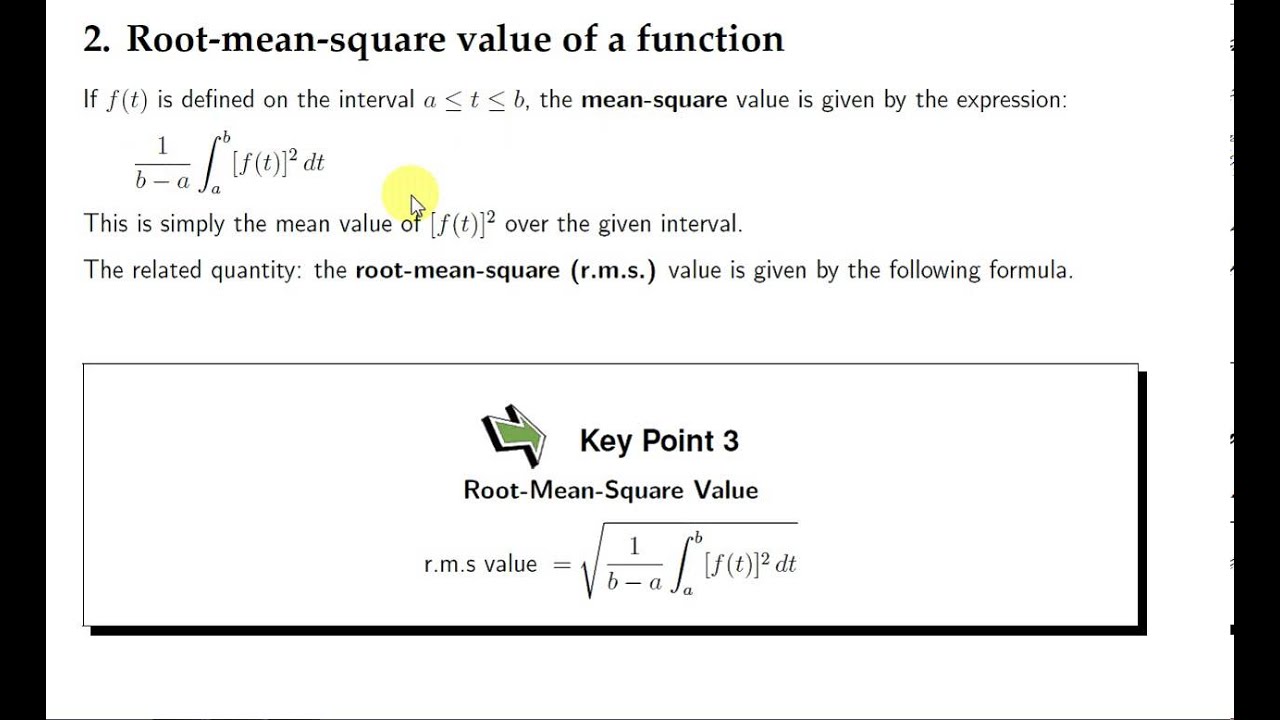
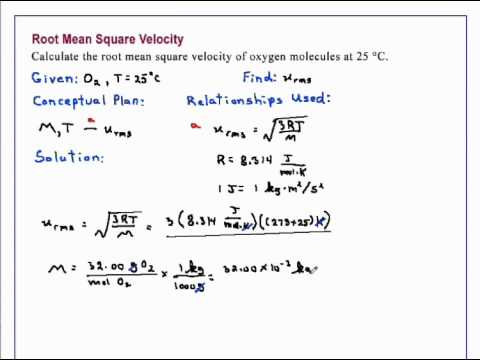
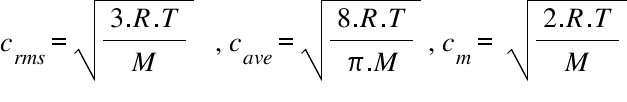At what temperature is the root mean square speed of nitrogen molecules equal to the root mean square of hydrogen molecules at 20 degree Celsius? - Quora

SOLVED: Calculate the root mean square velocity and kinetic energy of F2, Cl2, and Br2 at 298 K. Rank the three halogens with respect to their rate of effusion.

Calculate the temperatures at which the root mean square velocity, average velocity and most probable velocity of oxygen gas are all equal to 1500 ms^-1 :

How to Calculate the Root Mean Square Speed of Molecules in Gas at a Certain Temperature | Physics | Study.com

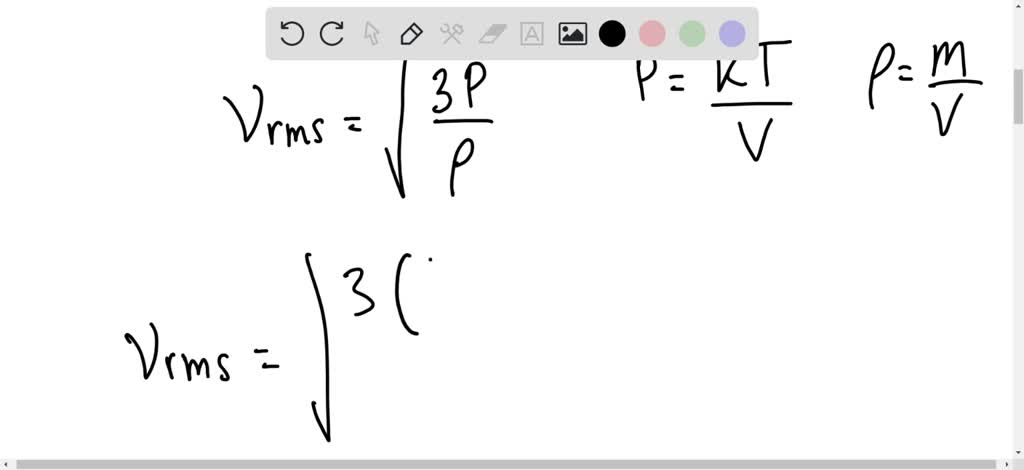
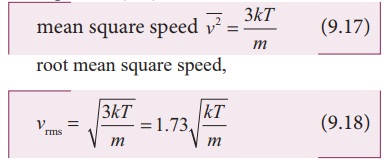
Calculate the rms velocity of molecules of a gas of density 1.5 g litre^(-1) at a pressure of 2 xx 10^(6) N//m^(2).