
Aqueous solutions of NMA, Na2HPO4, and NaH2PO4 as models for interaction studies in phosphate–protein systems - ScienceDirect

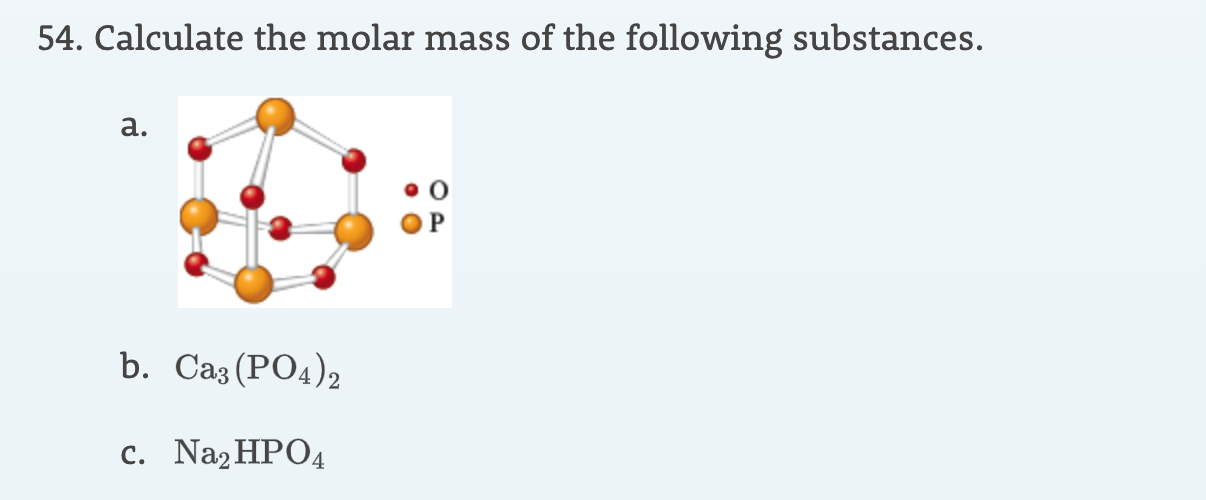
The percentage composition of sodium phosphate as determined by analysis is 42.1% sodium, 18.9% phosphorus and 39% oxygen. Find the empirical formula of the compound (work to two decimal places). (R.A.M: Na = 23, P = 31, O = 16.)

Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com
Q. The equivalent mass of H3PO4 (Molecular weight = 98 g/mol) and Na2HPO4 (Molecular weight = 142 g/mol) in the reaction are respectively : H3PO4 + 2NaOH → Na2HPO4 + 2H2O (1) 49, 142 (2) 49, 71 (3) 98, 71 (4) 98, 142
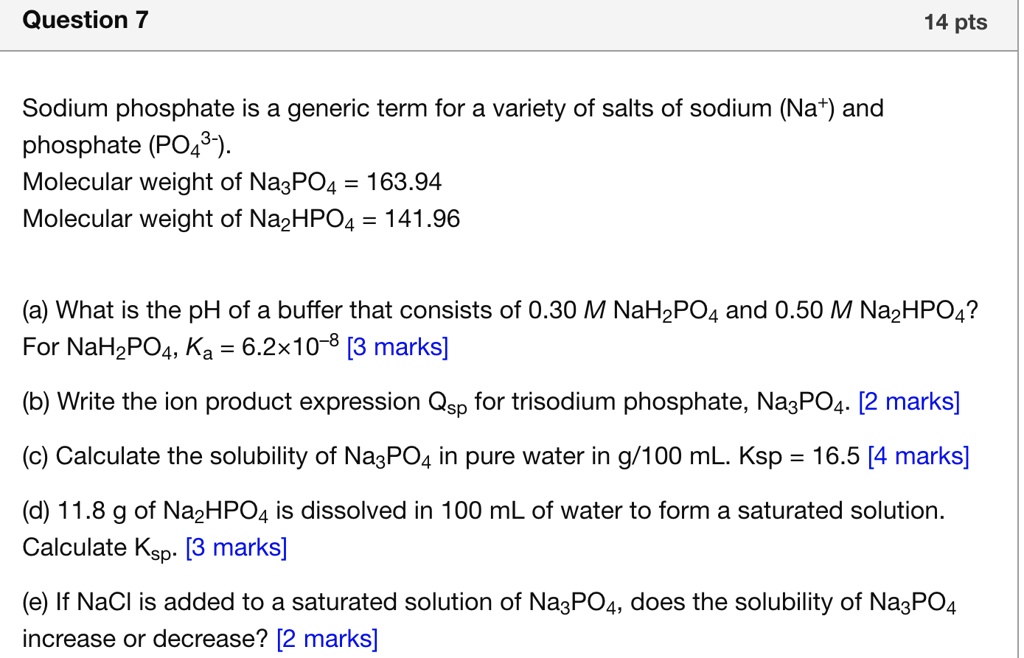
SOLVED: Question 7 14 pts Sodium phosphate is a generic term for a variety of salts of sodium (Nat) and phosphate (PO4 3-). Molecular weight of NazPO4 163.94 Molecular weight of NazHPO4
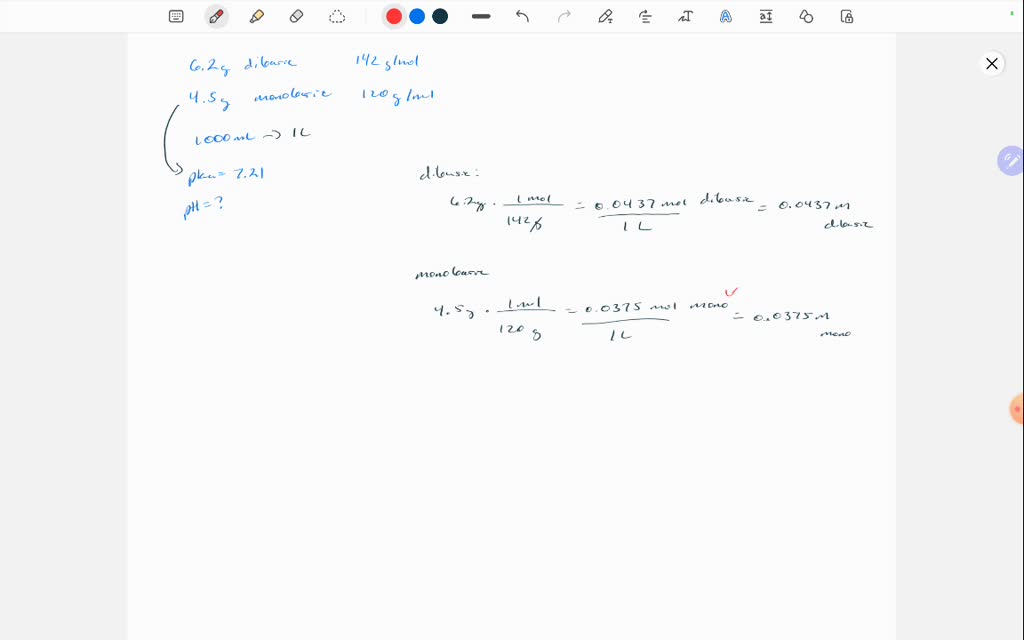
![SOLVED: 'Finally, what mass of Na2HPO4 is required? Again, assume a 1.00 L volume buffer solution. Target pH = 7.49 Acid/Base pair: NaH2PO4 Na2HPO4 PK = 7.21 [Na2HPO4] > [NaH2PO4] (NaH2PO4) = SOLVED: 'Finally, what mass of Na2HPO4 is required? Again, assume a 1.00 L volume buffer solution. Target pH = 7.49 Acid/Base pair: NaH2PO4 Na2HPO4 PK = 7.21 [Na2HPO4] > [NaH2PO4] (NaH2PO4) =](https://cdn.numerade.com/ask_images/0998c21f74d04a76817b4a340a0978cd.jpg)
SOLVED: 'Finally, what mass of Na2HPO4 is required? Again, assume a 1.00 L volume buffer solution. Target pH = 7.49 Acid/Base pair: NaH2PO4 Na2HPO4 PK = 7.21 [Na2HPO4] > [NaH2PO4] (NaH2PO4) =
Lab Manual Ch 2 (pg 31) 1. A) How would you prepare 500 mL of 0.1 M NaH2PO4 starting with the solid salt?

Comparative Study of Sodium Phosphate and Sodium Sulfate in Aqueous Solutions at (298.15 to 353.15) K | Journal of Chemical & Engineering Data

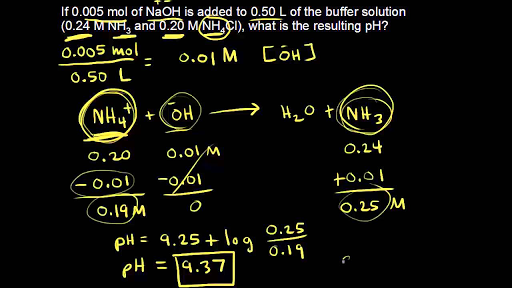
Finally, what mass of Na2HPO4 is required? Again, assume a 1.00 L volume buffer solution. Target pH = - Brainly.com
![Decane [C10H22] Molecular Weight Calculation - Laboratory Notes Decane [C10H22] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2022/10/decane-molecular-weight-calculation-300x194.jpg)






![Answered: [A-] pH= pka + log [HA] a. Determine… | bartleby Answered: [A-] pH= pka + log [HA] a. Determine… | bartleby](https://content.bartleby.com/qna-images/question/0efbec1c-3aeb-47c8-90fa-39b6de84bb74/c728bc90-2d81-4d3c-b78c-7dd9d14854f6/k04zzb.jpeg)